Pharmacodynamics
Extensive experience in pharmacodynamic bioanalysis and GLP/GCP quality assurance system
Bioanalytical
Pharmacodynamics
Kanwhish has rich experience in pharmacodynamic bioanalysis and GLP/GCP quality assurance system, providing preclinical/clinical bioanalytic services to support pharmacodynamic research related to regulatory filing.
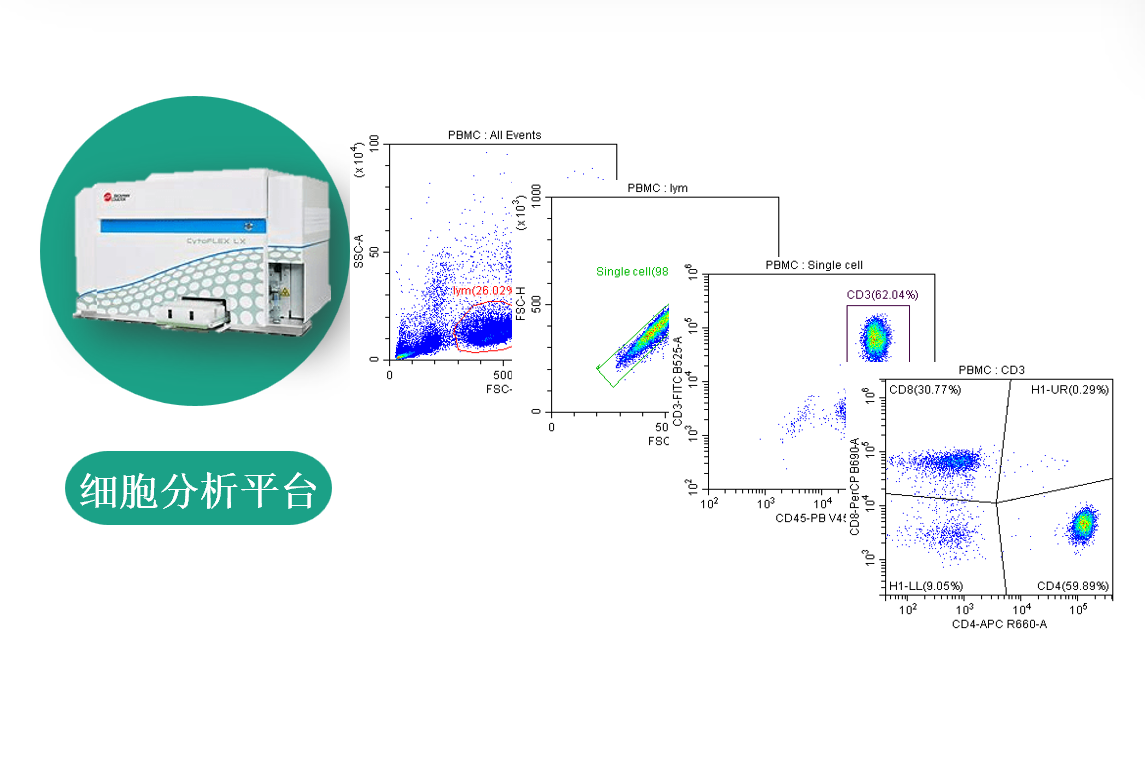
Over 95% of the analysis methods at Kanwhish Biotech are developed in-house. We offer customized, one-stop receptor occupancy (RO) analysis services, as well as single-factor or multiplexing cytokine analyses. Additionally, we can analyze protein expression from endogenous/exogenous gene in the field of cell and gene therapy.
| Customized services for pharmacodynamic biomarker analysis; |
| Experience in developing single or multi-target RO analysis methods; |
| Complete set of PD analysis instruments and equipment, including qPCR, FACS, MSD, Luminex etc. |
Please tell us your questions or needs
and Kanwhish Biotech will contact you as soon as possible via email or phone.

? 2022 - 2025 CopyrightAll Rights Reserved. ADD:江蘇省蘇州市吳中區(qū)尹山湖路999號(hào)C3幢4樓 蘇ICP備2023013734號(hào)-1
 ? 2022-2025 Copyright All Rights Reserved. 蘇ICP備2023013734號(hào)-1
? 2022-2025 Copyright All Rights Reserved. 蘇ICP備2023013734號(hào)-1